Every transfer is a test of strategy
By Staff Report June 20, 2025 6:25 pm IST
To remain competitive and ensure long-term growth, manufacturers must implement strategic transfers of lines and plants, much like a seasoned captain navigating through a storm amidst evolving dynamics. Dr. Narayanan Ramanathan, Chief Delivery Officer at L&T Technology Services, emphasises the importance of strategic transfers in the medical device sector to ensure long-term growth and adapt to changing market dynamics.
In a D-VUCA (Disrupted – Volatile, Uncertain, Complex, and Ambiguous) world, global manufacturers, in the medical device sector, face mounting challenges. From evolving regulations, geopolitical tensions, supply chain disruptions and fierce market competition to the need to stay competitive in a dynamic market multiple sink-or-swim scenarios exist.
To remain competitive and ensure long-term growth, manufacturers must implement strategic transfers of lines and plants, much like a seasoned captain navigating through a storm amidst evolving dynamics.
A well-thought-out transfer strategy does more than avoid short-term disruptions; it builds long-term resilience into the production ecosystem – helping global companies adapt to cost pressures, reduce regional risks, and position themselves closer to emerging markets and untapped opportunities. Such a decision can ensure stability, enable compliance, and drive sustainable growth.
Line and plant transfers
Line and plant transfers involve relocating or expanding manufacturing lines, assembly layouts, or entire production facilities to new locations. The focus is on minimising disruption to operations, ensuring compliance with regulatory standards, and enabling cost and efficiency optimisation.
For leaders in the medical device industry, the process can be particularly stringent and demanding due to regulatory requirements, including the FDA, EU MDR, and ISO 13485. The importance of precision, patient safety, and quality standards in manufacturing operations is crucial.
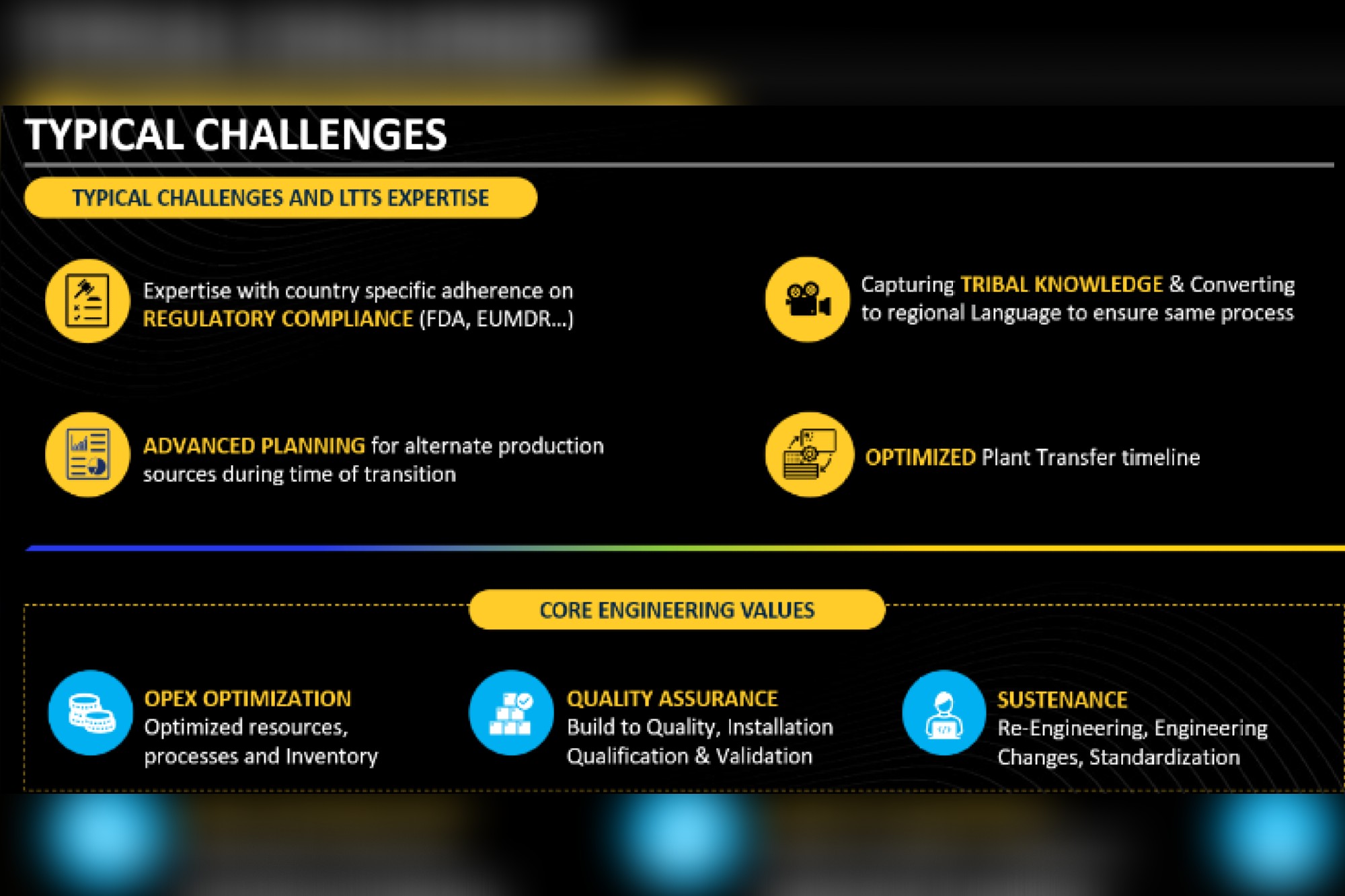
What is needed, therefore, is a balanced approach. It requires an emphasis on digital engineering, smart tooling and automation, seamless supply chain localisation, streamlined regulatory compliance, and robust sustainability. Only then can companies achieve seamless transfers that maintain and accelerate operational excellence.
Strategic considerations for line and plant transfer in manufacturing
For a manufacturer, successful line and plant transfers are driven by:
Cost Optimisation: Relocating to regions with lower operational costs while improving productivity and maintaining quality.
Capacity Expansion: Scaling production to meet the growing demand for medical devices, such as diagnostic equipment or wearables across diverse geographies.
Regulatory Compliance: Ensure new facilities meet global standards such as GMP and FDA 21 CFR PART 820.
Enabling supply chain resilience: To reduce risks and ensure proximity to key markets.
Sustainability: Design new plants for energy efficiency and rapid progress toward net-zero targets.
Technology Integration: Implement Industry 4.0 technologies, including IoT, AI, and digital twins, to modernise operations.
These strategic considerations are particularly crucial in the medical devices sector, where even minor disruptions can compromise patient care, delay regulatory approvals and damage brand reputation.
As a global engineering services provider with deep expertise in plant and line transfers, they realise that a successful transfer strategy needs to balance each of these objectives. The transfer process must minimize downtime, ensure workforce readiness, and maintain overall product quality.
A tested approach to successful line and plant transfers
They leverage industry-leading expertise in plant engineering, digital manufacturing, and medical device engineering to deliver tailored line and plant transfer solutions for global clients across industries and geographies. LTTS’ framework for successful transfers consists of four key pillars: product integration, process integration, plant integration, and people integration.
Robust execution framework
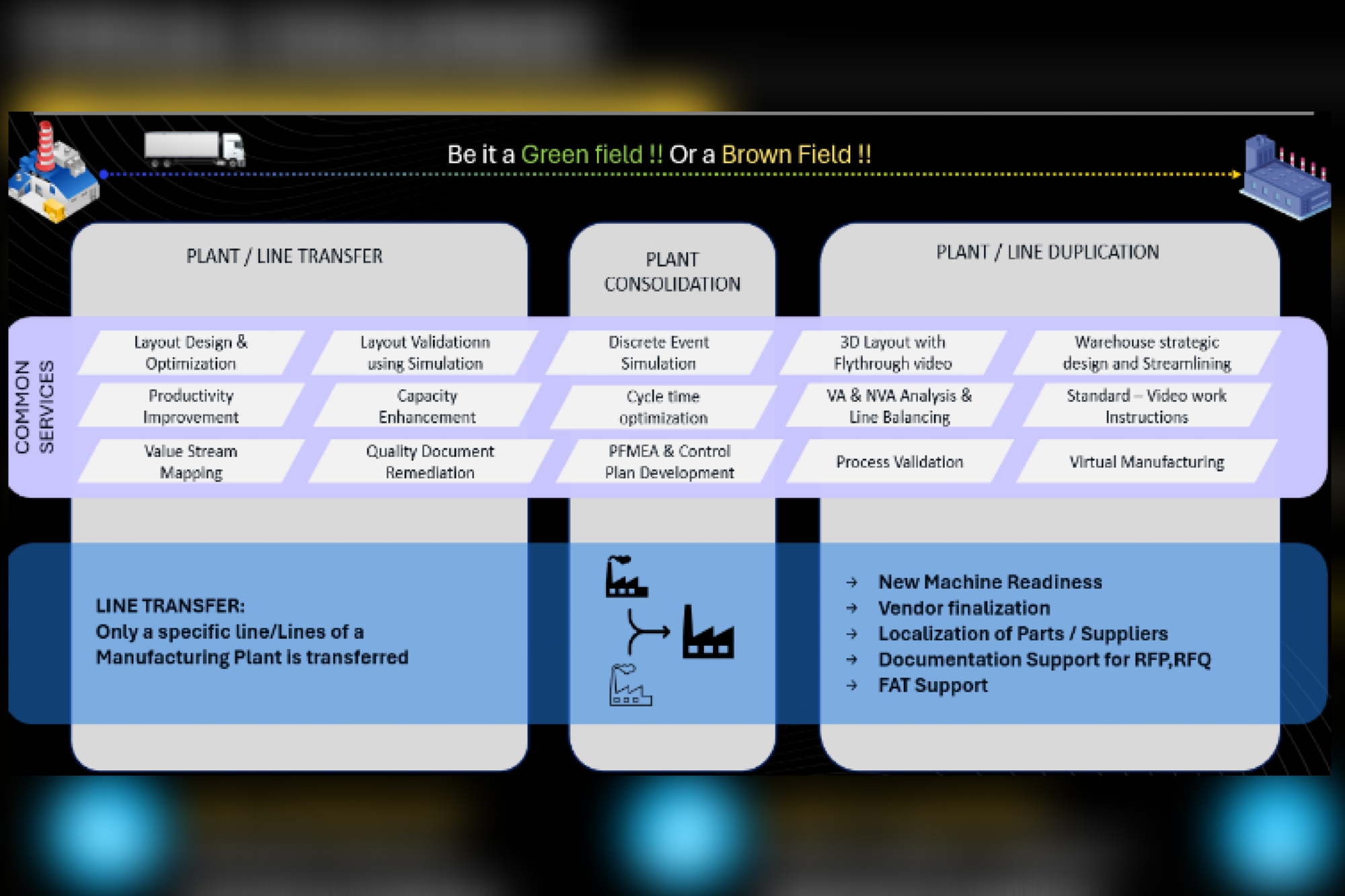
LTTS’ comprehensive framework for handling end-to-end plant transfer and consolidation projects is divided into three phases, consisting of eight constituent steps.
Pre-transfer phase: This involves three steps – As-Is Study and Transfer Planning, Detailed Engineering, and Pre-Dismantling.
Transfer phase: Comprises two steps – Dismantling and Commissioning Readiness.
Post-transfer phase: Follows three steps – Installation and Commissioning, Process Qualification, and Sustenance.
Digital engineering and industry 4.0 integration
LTTS is utilising digital twins, AI, IoT, and generative AI to improve transfer efficiency and future-proof new facilities. Digital twins simulate plant layouts and processes to identify bottlenecks before physical transfer, while AI-powered automation streamlines high-volume tasks like clinical evaluation report processes. LTTS’ UBIQWeise IoT platform enables remote monitoring of equipment health, reducing downtime during transfers. Generative AI, partnered with hyperscalers like AWS and NVIDIA, optimises manufacturing processes across smart vehicles and medical devices.
LTTS technology stack helps ensure that the new plants are equipped with smart manufacturing capabilities, including real-time quality control for diagnostic devices.
Workforce enablement and change management
Transferring a plant requires upskilling local teams and ensuring operational readiness. The proposed approach includes:
Training and simulation: Using ergonomic simulations and AR/VR to train workers on new processes to ensure a 100% safe environment.
Knowledge transfers: By effectively capturing the tribal knowledge, digitalisation requirements and localised training content to enable a team with no prior experience to operate effectively, and
Change management: Aligning global stakeholders to drive adoption and reduce resistance.
LTTS has demonstrated capabilities in training teams on handling sophisticated equipment, including AI-powered radiology tools and endoscopic platforms.
Enabling sustainability and cost efficiency
LTTS focuses on sustainability and cost-efficiency in plant and line transfer lifecycles. They use IoT-based platforms to optimise energy consumption, design lean layouts, reduce cycle time by up to 20%, and enable predictive maintenance using AI-powered platforms. This approach reduces operational expenditure and minimises unplanned downtime, which costs manufacturers over $50. The company’s process optimisation also reduces manufacturing cycle times by 20%.
Harnessing a Proven Track Record
LTTS has a proven track record in cross-industry line and plant transfers across industries, with notable examples relevant to leading global medical device firms, including:
A USD 100 million+ plant engineering engagement: partnered with a global O&G leader on two US-based refining and chemical facilities, delivering integrated engineering and operational efficiency over 5 years.
Cutting-edge medical device engineering: LTTS helped a European ultrasound market leader design next-generation scanners, integrating AI and cloud connectivity.
Partnerships with tech leaders: LTTS collaborates with Siemens, Rockwell, AWS, NVIDIA, and Google Cloud to deliver cutting-edge solutions, enhancing its transfer capabilities.
LTTS’s recognition as a Leader in Connected Medical Device Services by Everest Group (2022) and its ranking among the Top 3 Global Pure Play ESPs in PLM Services underscore its expertise in medical and plant engineering.
Tailored recommendations for a medical major
In light of global trends, medical device leaders should consider the following phased approach for effective line and plant transfer:
Adopt a Phased Approach:
Phase 1 involves digital planning using digital twins to align layouts and processes with FDA, ISO 13485, and EUMDR requirements. Phase 2 uses concurrent engineering to accelerate equipment transfer without compromising quality or compliance. Phase 3 integrates AI, and IoT for real-time monitoring and maintenance.
Prioritize Regulatory Compliance:
Partner with a trusted engineering and technology partner to implement traceability systems and automate documentation (e.g., CER processes) to streamline audits and ensure audit readiness.
Deploy HL7, and DICOM integrations to ensure seamless interoperability with EMR systems and compliance with clinical workflows.
Leverage digital technologies
Utilise digital technologies like AI for quality control and process optimisation and AR/VR training modules for workforce readiness and reduced onboarding time for complex medical devices.
Focus on Sustainability
Implement platforms like LTTS iBEMs to optimise energy usage, identify high-consumption zones and drive measurable reductions in carbon emissions.
Strengthen supply chain resilience through predictive demand planning, alternate sourcing models and regional vendor integration.
Conclusion
An effective line and plant transfer strategy must take a holistic, technology-driven approach anchored in digital engineering, regulatory compliance, and sustainability. Seamless transfers that minimize disruptions, reduce costs, and maximise operational efficiency are powered by enablers like AI, IoT, and digital twins. Achieving this demands a global delivery model built on engineering depth, execution, agility and regulatory rigour, ensuring consistent outcomes across geographies.
In today’s turbulent environment, leadership means steering with clarity, ensuring your operations remain resilient, compliant, and future-ready.
Cookie Consent
We use cookies to personalize your experience. By continuing to visit this website you agree to our Terms & Conditions, Privacy Policy and Cookie Policy.